Clinical relevance of oxygenation and perfusion
Tissue oxygenation, perfusion, or a combination of both metrics are closely tied to the mechanisms of numerous diseases.
In ischemia, blood vessels become narrowed or fully occluded, often due to chronic lipid accumulation in blood vessel walls. This restriction of blood supply deprives tissues of oxygen, resulting in overall reduced perfusion in microvasculature as well as reduced oxygenation in the tissues of interest. Peripheral arterial disease (PAD) is an example of a major disease involving ischemia and can lead to severe health consequences without proper diagnosis and disease management.
On the other hand, inflammation is associated with increased perfusion and oxygenation in affected tissues. Under normal circumstances, inflammation is a protective response by the body to increase blood supply in an area of damaged tissue. However, chronic inflammation has adverse effects for tissue health and patient well-being, as seen in diseases like inflammatory bowel disease (IBD) and arthritis. Additionally, inflammation is an early disease mechanism in some neuromuscular diseases (such as Duchenne muscular dystrophy).
Beyond perfusion and oxygenation, other conditions relating to vasculature and hemoglobin are commonly encountered in cancer, such as the formation of new blood vessels via angiogenesis or tumor hypoxia resulting from rapid cell division and tissue growth.
![]()
The limitations of established clinical diagnostics
Owing to the central role that tissue perfusion and oxygenation play in these disease areas, clinical tools that report on these metrics benefit patient care at all stages – including diagnosis, disease management, choice of therapy, and triggers for timely intervention.
Established methods are available to assess superficial microperfusion in the skin, while others can assess arterial macroperfusion in large blood vessels. In current clinical practice, widely used methods include transcutaneous oxygen monitoring (TcPO2) and skin perfusion pressure (SPP) to assess superficial structures. Deep measurements of large blood vessels can be performed with the ankle brachial index (ABI), computed tomography angiography (CTA), magnetic resonance angiography (MRA), and duplex ultrasound (DUS). Other methods have also been explored in recent years, such as near-infrared spectroscopy (NIRS), contrast-enhanced ultrasound (CEUS), and blood-oxygen level dependent (BOLD) MRI, although these methods so far have not seen adoption in routine clinical use.
However, while the existing clinical toolbox covers skin micro- and arterial macroperfusion well, it lacks robust methods to understand musculoskeletal tissue perfusion and oxygenation. It should also be noted that perfusion and oxygenation in deep tissue cannot reliably be inferred from the skin or large blood vessels. Additionally, spectroscopic methods like NIRS cannot provide depth-dependent information, leading to significant variations (even within the same patient) depending on which exact region of the body is measured. This results in a critical diagnostic gap for (micro)perfusion and oxygenation at penetration depths beyond the first 1-2 mm of skin, leaving tissues like skeletal muscle and major organs largely out of scope for these technologies.
For example, in PAD, the oxygenation of leg muscle is of particular importance to diagnose and stage the disease, track progression, and gauge treatment success. However, available tests are difficult to interpret and may lead to undetected disease progression while a patient is asymptomatic – especially for patients who present with vascular calcification (which interferes with routine testing) or with diabetes (a common comorbidity of PAD), who often experience sensory loss due to neuropathy. The success of revascularization surgery is currently assessed via angiography, although this only delivers information on the degree of stenosis in large arteries and can’t directly report if muscle tissue oxygenation has returned to normal values following the removal of an arterial plaque. Here, a better understanding of muscle oxygenation can facilitate an optimized treatment choice (conservative vs. intervention), avoid PAD progression to severe stages (e.g., ulceration and necrosis, which eventually necessitate amputation), or predict the onset of disease as a comorbidity in diabetes patients.
Significant diagnostic gaps also exist for assessing inflammation in the bowel wall in IBD. In the current clinical gold standard, disease status and treatment success are assessed via endoscopy – a highly invasive procedure that puts a sizeable burden on the patient and carries added risk of tissue perforation for patients with inflamed bowels. Moreover, due to this risk and burden, endoscopic examinations cannot be safely performed with high frequency for continuous disease monitoring. On the other hand, clinical scores are easy to obtain but limited by their subjectivity. Thus, there is a major unmet need for non-invasive, low-burden, quantitative methods to report on inflammation and enable timely treatment adjustments in IBD.
Similarly, current assessment of inflammation in arthritis has limitations. In clinical practice, arthritic patients are evaluated via clinical examination as well as blood tests. While the former can be subjective, either from the perspective of the clinician or the patient, the latter can be non-specific and lack localization. Imaging approaches are also employed; X-ray and MRI can be used to assess joints directly (typically by evaluating the distance between the bones in a joint), and ultrasound can be used to assess edema. However, these established imaging approaches cannot directly report on inflammation, leaving a major diagnostic gap for assessing a central disease mechanism in arthritis.
![]()
Assessing deep tissue oxygenation and perfusion with MSOT
Ongoing research at the cutting edge of biomedical imaging has led to the recent development of a new diagnostic imaging modality – multispectral optoacoustic tomography (MSOT). This non-invasive imaging technology visualizes the concentration and distribution of various clinically important biomolecules, based on their natural light-absorbing properties.
Using MSOT, the tissue of interest is illuminated with light in the near-infrared range. Natural chromophores (such as hemoglobin) absorb photonic energy, which is then converted into heat and ultrasonic waves via the photoacoustic effect. Thus, MSOT functions on the principle of “light in, sound out”, with different biomolecules having different characteristic absorption profiles that enable detection and identification of specific disease biomarkers.
In the context of assessing oxygenation and perfusion, MSOT addresses several diagnostic deficiencies of other established technologies. Chiefly, MSOT enables non-invasive, quantitative spectral assessment of tissues at depths of up to 2 cm. MSOT imaging (as opposed to optical spectroscopy) delivers depth information, which facilitates precise localization of regions of interest. Lastly, owing to the unique absorption profiles of oxygenated and deoxygenated hemoglobin, MSOT reports on both the bulk hemoglobin signal (measure of tissue perfusion) as well as the fraction of oxygenated hemoglobin (measure of tissue oxygenation).
![]()
Demonstrated clinical potential of MSOT
Based on these unique properties, MSOT has been demonstrated as a potentially invaluable tool in numerous clinical applications, where the technology may be able to bridge long-standing diagnostic gaps.
In PAD, muscle tissue ischemia is both a central disease mechanism as well as a biomarker of disease status. MSOT has been explored as a novel tool for PAD diagnosis, staging, and evaluation of treatment success. A recent study performed at the University Hospital Erlangen reported that MSOT signal could be used as a robust classifier to distinguish healthy volunteers from PAD patients. Furthermore, well-controlled muscle provocation by means of a heel raise at the bedside was shown to further improve the diagnostic accuracy.
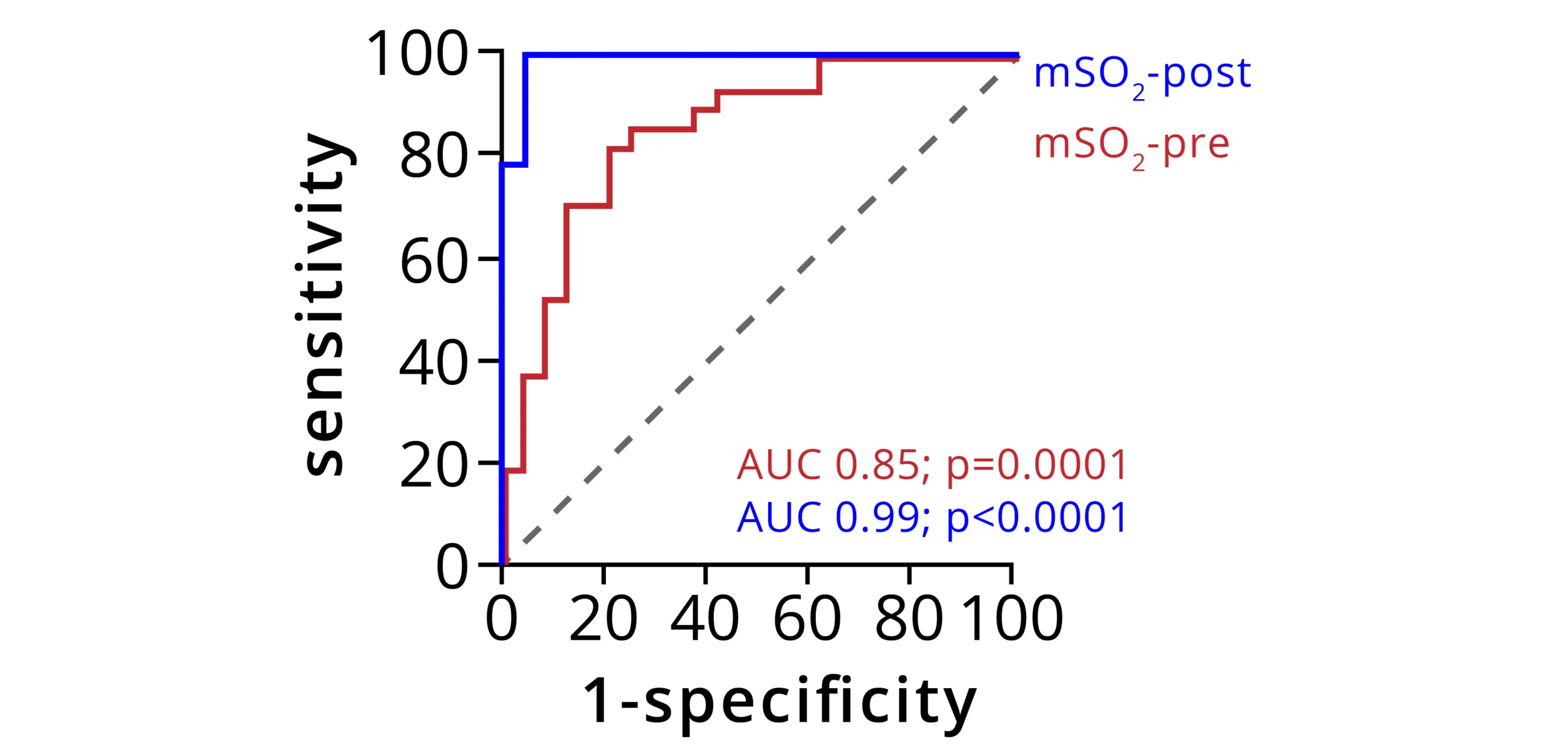
Receiver operating characteristic (ROC) curve analysis of MSOT signal to classify participants before exercise (mSO2-pre, red curve) or after exercise (mSO2-post, blue curve). Area under the curve (AUC) was calculated with a 95% confidence interval. Dashed line indicates the random classifier line of no discrimination. Data adapted from: Derivation and validation of a non-invasive optoacoustic imaging biomarker for patients with intermittent claudication. medRxiv 2023.10.19.23297246 (2023)
Lower extremity revascularization (LER) is a surgical or minimally invasive intervention employed to treat late-stage PAD. In LER, narrowed or obstructed blood vessels are mechanically opened by either a bypass or by insertion of a balloon catheter, atherectomy, or stent to reestablish blood supply in affected tissue. Follow-up assessments are conducted to determine if the surgery was successful or if occlusions are still present and reintervention is necessary. To that end, angiography (CTA or MRA) is currently employed to image large blood vessels and identify stenoses. However, angiography does not report on perfusion or oxygenation of affected muscle tissue, where reduced microperfusion and the resulting ischemia leads to the clinical symptoms of intermittent claudication and rest pain.
In this context, MSOT has been investigated as a potential tool for assessing the outcome of LER by perioperatively measuring muscle tissue perfusion and oxygenation before and after an interventional procedure. Researchers at the Technical University Munich and University Hospital Münster found that MSOT was able to detect a significant difference in muscle tissue oxygenation in PAD patients before and after LER. Additionally, following LER, the oxygenated hemoglobin concentrations of PAD patients returned to similar values observed in healthy volunteers. Thus, MSOT could serve not only as a follow-up diagnostic, but also perioperatively as a tool to confirm in real time whether tissue oxygenation has been reestablished.
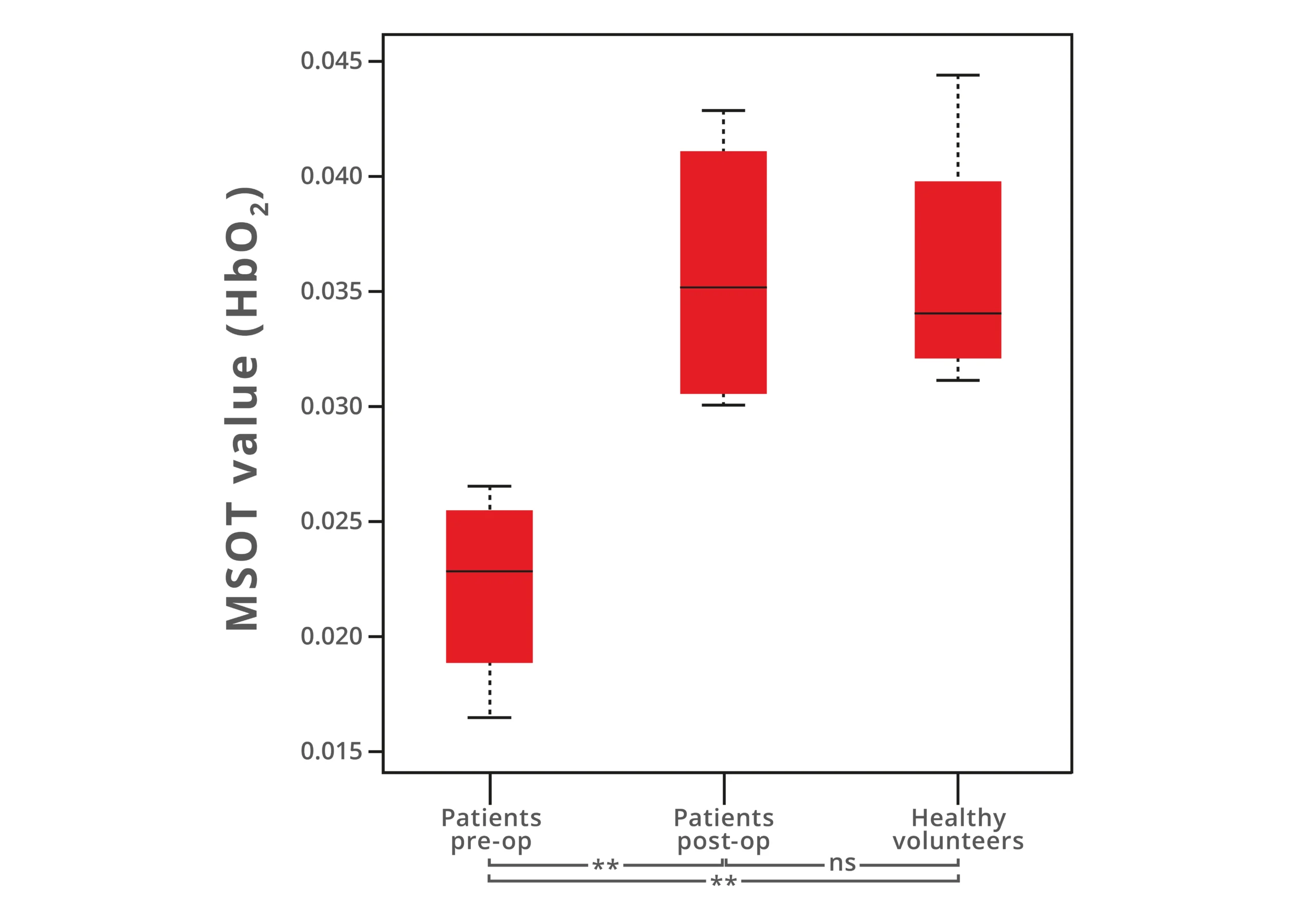
MSOT measurements comparing oxygenated hemoglobin concentrations (HbO2) in PAD patients before (pre-op) and after (post-op) LER intervention as well as to healthy volunteers. Data adapted from: Multispectral optoacoustic tomography of peripheral arterial disease based on muscle hemoglobin gradients - a pilot clinical study. Ann Transl Med. (2021)
MSOT has also been explored as an innovative diagnostic approach to assess inflammation in the bowel wall, using hemoglobin concentration as a biomarker of inflammation. In a pilot study performed at the University Hospital Erlangen, perfusion in the bowel wall of IBD patients in various disease stages (from remission to high active inflammation) was examined with MSOT, with significant differences observed between the different disease stage groups. Thus, the degree of perfusion may help distinguish between patients in remission who have responded well to treatment and those with active relapse. This measure of treatment success could be an invaluable aid for clinicians to optimize therapy and possibly reduce the need for the highly invasive procedure of endoscopy.
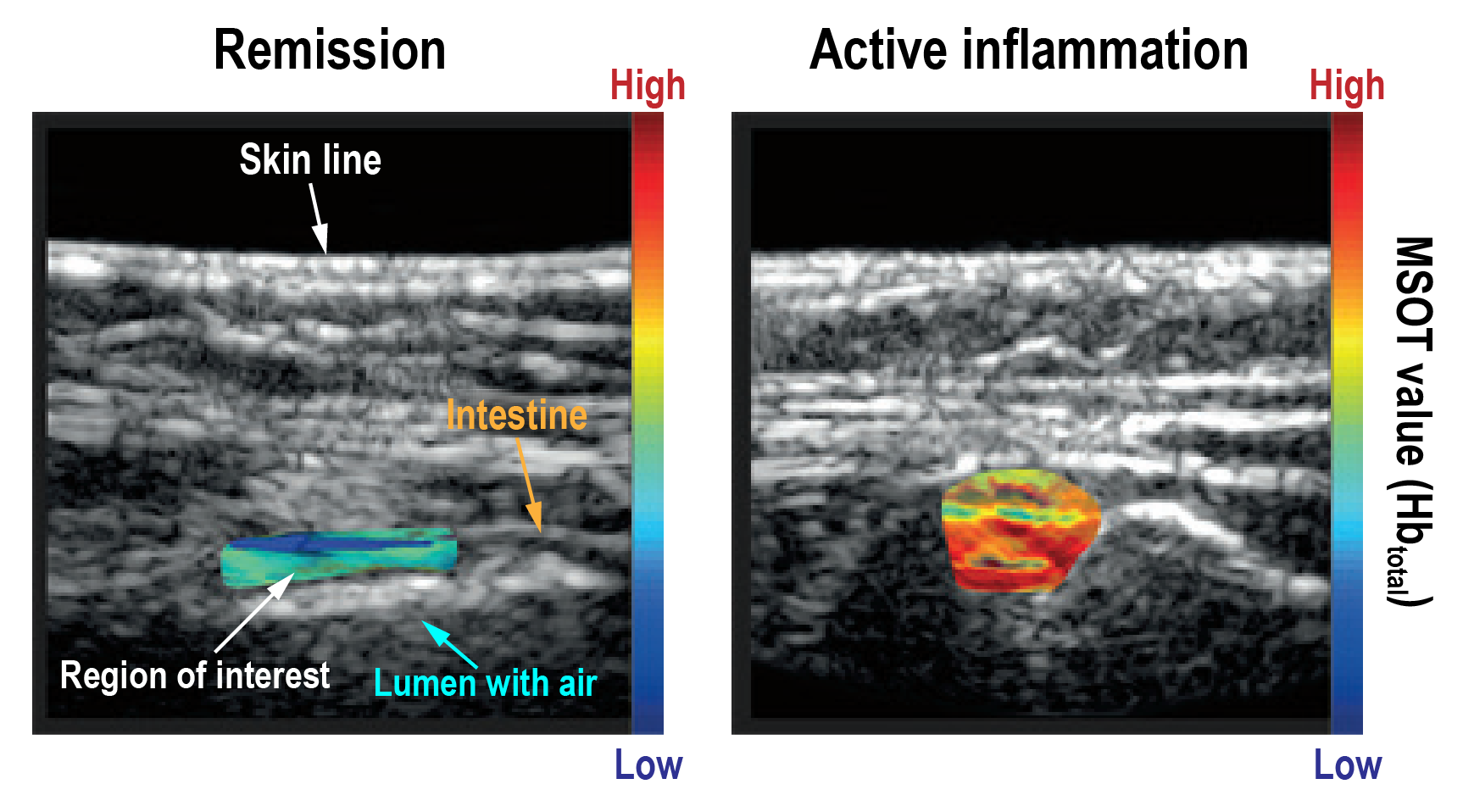
Representative images of the large bowel and small intestine in IBD patients in remission (left) and with active inflammation (right), as staged via endoscopy. MSOT data of total hemoglobin (color) is overlaid on B-mode ultrasound (grayscale). Data adapted from: Multispectral optoacoustic tomography for assessment of Crohn’s disease activity. N Engl J Med. (2017)
In current clinical assessment of arthritis, established methods are unable to directly measure tissue inflammation. To bridge this diagnostic gap, researchers at the University Hospital Essen explored the feasibility of using MSOT as an assessment tool to quantify inflammation. Here, they measured MSOT-derived signals of oxygenated and deoxygenated hemoglobin and found statistically significant differences between arthritic patients and the healthy volunteer control group. These results suggest that MSOT may be able to offer direct, objective patient assessment and facilitate timely, accurate diagnosis of arthritis.
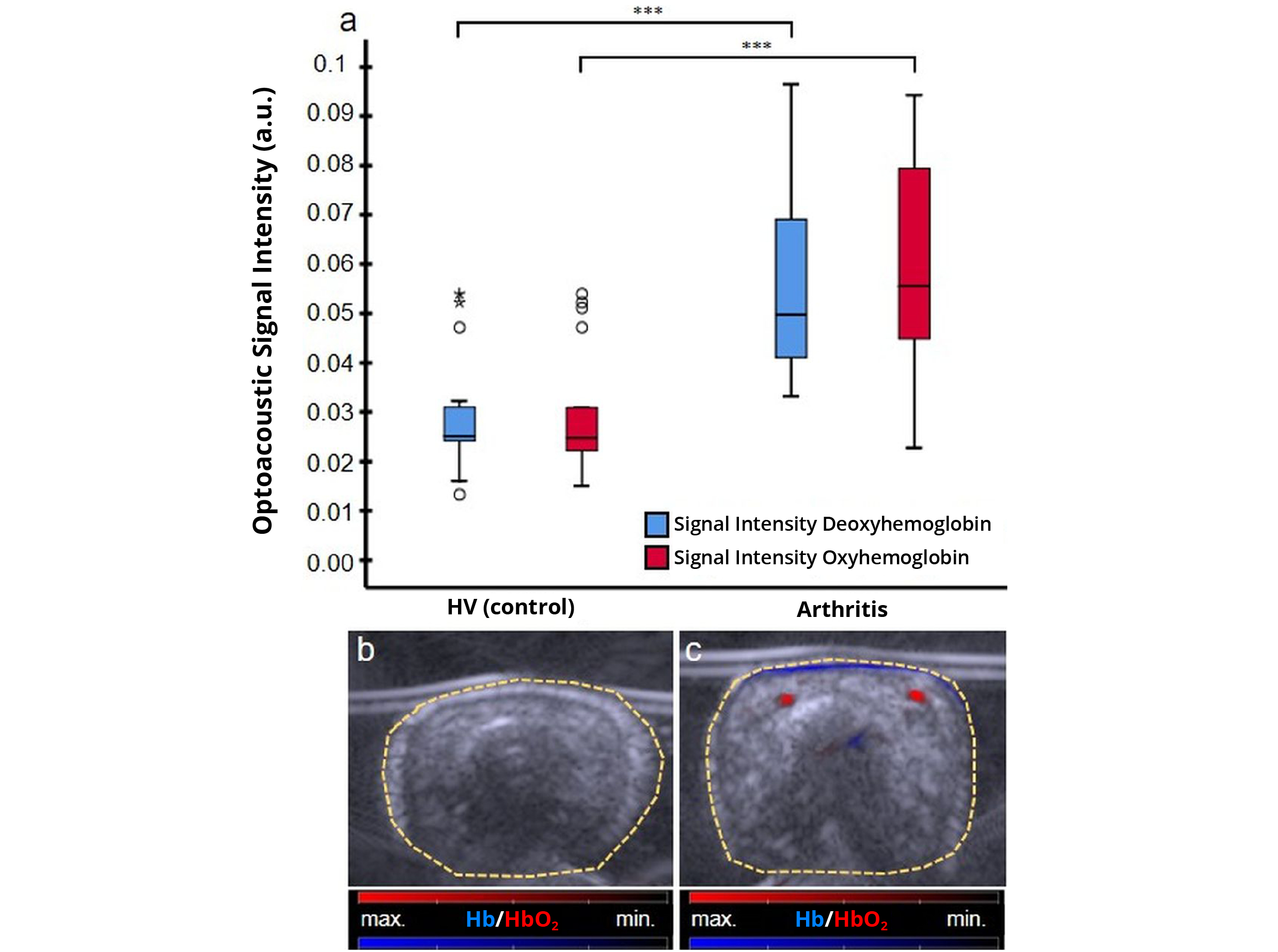
MSOT measurements comparing the oxygenated (HbO2, red) and deoxygenated (Hb, blue) hemoglobin signals in patients with psoriatic arthritis and healthy volunteers (HV). Data adapted from: Multispectral optoacoustic tomography might be a helpful tool for noninvasive early diagnosis of psoriatic arthritis. Photoacoustics (2020)