The circulatory system is vital to supply tissues with oxygen and blood-delivered nutrients, and disruptions of this function can have serious medical consequences. In peripheral arterial disease (PAD), chronic restriction of blood supply (i.e., ischemia) to the extremities can cause a wide range of symptoms depending on the severity of the condition and the affected area of the body. While most cases are asymptomatic, more severe cases can lead to muscle pain, atrophy, numbness, ulceration and, in extreme cases, gangrene or necrosis – possibly requiring amputation.
PAD (in all forms) is relatively widespread and is estimated to affect around 5% of the world's population, with an exceptionally high prevalence of 20% in people over 70 years of age. Beyond the physical burden for individual patients, the worldwide healthcare costs associated with the assessment, treatment, and management of PAD are estimated at €100 billion per year worldwide, causing a significant financial strain that may be reduced through improved disease management.
![]()
The need for robust PAD assessment
Early detection of PAD and disease progression monitoring are crucial to enable timely intervention (e.g., with medication) or the application of preventive and risk-reducing measures. Additionally, a better understanding of the precise pathological changes in affected tissue can support better clinical decision-making for treatment. Despite the need for approaches to accurately monitor and diagnose PAD, there are currently no established, widely applicable methods to assess muscle tissue perfusion and oxygenation. Moreover, the extent of muscular damage that may occur from chronic mal-perfusion is currently only quantifiable through tissue biopsy and histological analysis.
Previous clinical studies have demonstrated that Multispectral Optoacoustic Tomography (MSOT) can derive metrics of tissue hemoglobin oxygenation, which is an potential biomarker for staging PAD. Although multispectral optoacoustic imaging can deliver detailed information on specific biomolecules, simplified optoacoustic imaging parameters (such as single-wavelength imaging) may provide similarly valuable clinical information on muscular damage occurring in PAD - especially when combined with an established modality like ultrasound imaging.
![]()
Hybrid optoacoustic-ultrasound imaging
In a recently published study led by researchers at University Hospital Erlangen, investigators explored the incremental clinical benefit of the use of hybrid optoacoustic-ultrasound imaging to visualize and quantify structural and functional muscular changes in PAD patients.
To that end, they utilized a simplified approach with a single optoacoustic wavelength at 800nm in concert with ultrasound imaging to differentiate patients according to disease severity. The performance of the single-wavelength bulk signal at 800nm was found to be comparable to that of the unmixed signals of tissue oxygenation obtained through multispectral imaging, with both approaches able to distinguish healthy volunteers from PAD patients. Furthermore, disease classification via multiple logistic regression combining single-wavelength signal at 800nm and ultrasound grayscale levels outperformed classification using only ultrasound grayscale levels, improving diagnostic accuracy of using ultrasound alone from 74% to 88% when using MSOT in combination with other clinical parameters.
They also investigated muscular morphological changes resulting from chronic ischemia. Histological analyses showed an increase in extracellular collagen in PAD patients, indicating fibrosis and muscle degeneration in affected tissues. As the single-wavelength optoacoustic signal at 800nm maps tissue morphology (as opposed to tissue oxygenation reported by multispectral optoacoustic imaging), and the hybrid optoacoustic-ultrasound approach was able to distinguish healthy from diseased tissue, this may provide a means to visualize the extent of muscle damage caused by PAD.
![]()
The potential clinical value of MSOT in PAD management
The unmet clinical need for non-invasive, quantitative methods to assess end organ damage occurring in PAD is a pressing issue for the management and treatment of the disease. Earlier detection of extensive muscle damage may enable identification of high-risk patients and prompt timely interventions, thus avoiding extreme outcomes (such as the need for amputation).
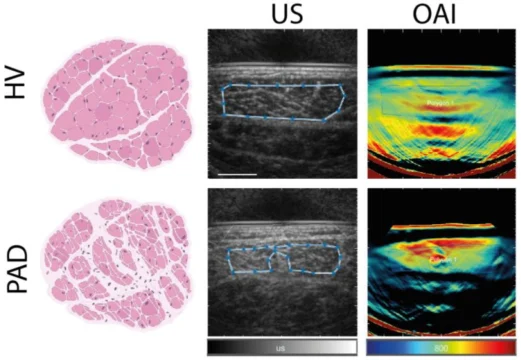
Left: Illustrations of healthy muscle tissue from a healthy volunteer (HV) and fibrotic tissue from a PAD patient. Center: Representative grayscale ultrasound (US) images from a healthy volunteer and a PAD patient. Right: Representative optoacoustic imaging (OAI) signals at 800nm from a healthy volunteer and a PAD patient.