Pompe disease – Pathology and cutting-edge therapy
Pompe disease (PD) is a metabolic disorder originating from deleterious mutations in the gene encoding a critical enzyme, acid alpha-glucosidase (GAA). This enzyme is required to catabolize the glucose storage molecule glycogen, which is primarily sequestered in the liver and skeletal muscle. However, patients with PD exhibit a significant deficiency in GAA activity, resulting in the buildup of unmetabolized glycogen in these cells and leading to widespread damage to muscle and nerve cells.
For patients with PD, recent decades have seen the development of several pharmacological treatments in the form of enzyme replacement therapy (ERT) that can significantly improve patient outcomes and quality of life. Here, a recombinant analog of the GAA enzyme is administered in regular intervals, thus compensating for the lack of endogenous GAA activity and allowing for improved glycogen metabolism.
![]()
The need for better diagnostic approaches to monitor disease severity
Notably, ERT is not curative. Lifelong infusions of the analog enzyme are required to manage disease symptoms. Moreover, patients must be closely monitored for therapy response, thereby necessitating robust diagnostic methods that are able to accurately report on disease status to allow for informed clinical decisions regarding treatment.
In current clinical practice, few methods have been established to quantitatively assess PD severity or therapy response to ERT. The gold standards for monitoring PD status and treatment response are clinical scores and functional tests – both of which are subjective, relying on the judgement of the clinician and cooperation of the patient. Moreover, as functional tests require that patients attempt to perform specific physical activities, these tests are inappropriate for infants, very young children, and advanced cases. Therefore, these tests often do not provide a strong foundation on which to assess disease status and therapy response.
Studies using magnetic resonance imaging (MRI) in late-onset PD patients have shown strong correlations between disease biomarkers and ERT efficacy as well as identified typical disease progression patterns. However, MRI as a method is challenging for infants and young children, as it requires the patient to remain motionless and thus may necessitate sedation. Additionally, patients with respiratory impairment (a common symptom in PD, due to weakness in the muscles controlling respiration) may find the positioning in the machine to be uncomfortable. This leaves an unmet need for non-invasive techniques that incur a low patient burden and are applicable for patients of all ages and disease stages.
![]()
Assessing muscle degeneration via optoacoustic imaging
As shown previously in numerous independent clinical studies, multispectral optoacoustic tomography (MSOT) is a novel clinical imaging approach that is able to detect anatomical, functional, and morphological changes in muscle tissue stemming from various neuromuscular diseases. The technology offers several advantages over many established diagnostic methods, such as quantitative assessment, non-invasiveness, and low patient burden. In that same vein, researchers at the University Hospital Erlangen explored the use of MSOT to image disease pathology and detect muscle degeneration in PD patients.
In this pilot study, ten PD patients of various ages and disease stages were included along with ten age-matched healthy volunteers. For each participant, the biceps muscle in the upper arm was assessed using MSOT as well as the established clinical diagnostics of ultrasound and MRI.
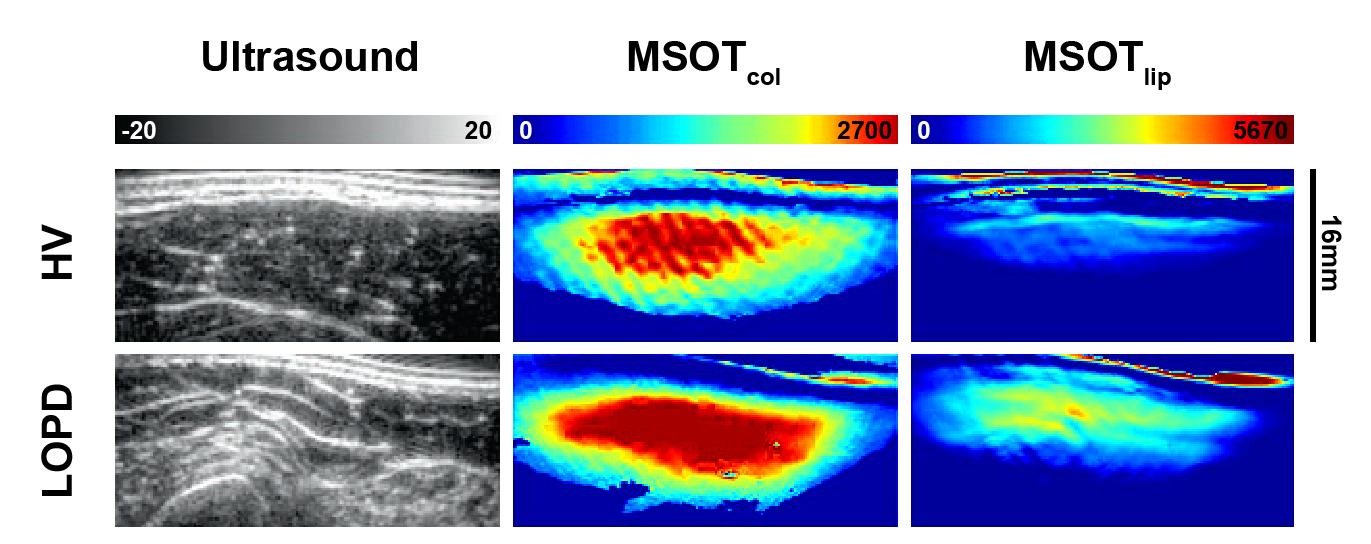
Representative images from scans of the biceps muscle of a participant from each cohort. Left to right: Ultrasound images, MSOT-derived collagen signal, and MSOT-derived lipid signal. Top to bottom: Healthy volunteer (HV) and late-onset PD (LOPD). Data adapted from Nat Commun. 2024 Sep;15(1):7843.
In many disorders involving muscle degeneration (such as neuromuscular diseases), a key underlying pathological process is fibrosis – the replacement of normal functional tissue with connective tissue, similar to scarring. This fibrotic tissue exhibits increased concentration of collagen compared to healthy muscle tissue, making collagen potentially a critical biomarker of muscle degeneration in these diseases. Similarly, increased lipid concentration is another biomarker of later-stage muscle degeneration in the context of fatty replacement. Thus, these two biomarkers presented particularly valuable targets for quantitative assessment.
To investigate the utility of MSOT as a potential diagnostic modality for PD patients, MSOT-derived signals attributed to collagen and lipid were obtained through multispectral imaging and spectral unmixing to determine the relative distribution of each biomolecule. Measurements obtained from healthy volunteers and pooled PD patients showed statistically significant differences between the two cohorts, indicating that the MSOT-derived signals may provide a means to detect and quantify the degree of muscle degeneration. Notably, quantification of relevant regions of interest from ultrasound data as well as MRI data did not display significant differences between the two groups.
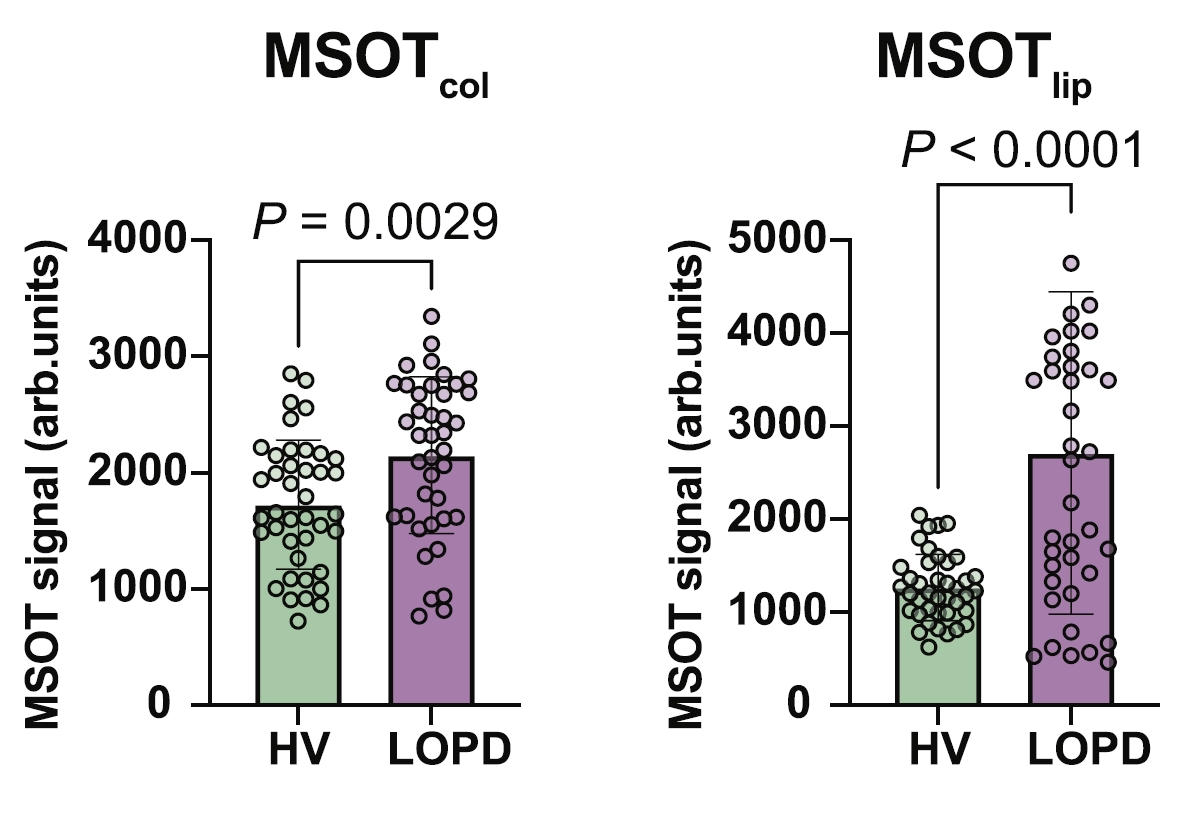
MSOT-derived collagen signal (left) and MSOT-derived lipid signal (right) from healthy volunteers (HV) and late-onset PD (LOPD) patients. MSOT signals are quantified in arbitrary units.
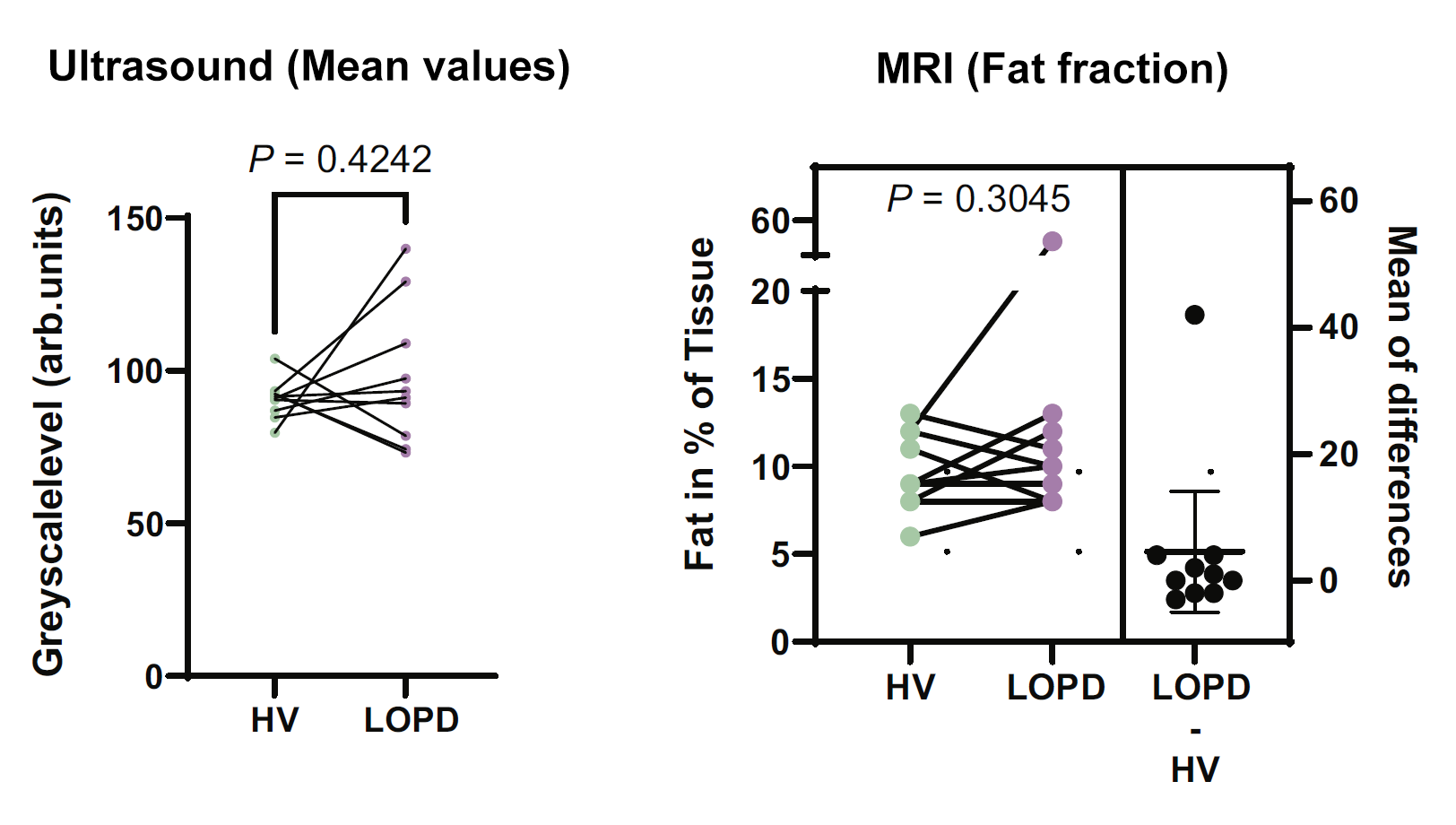
Ultrasound signal mean values (left) and MRI-derived fat fraction values (right) from healthy volunteers (HV) and late-onset PD (LOPD) patients. Ultrasound signals are quantified in arbitrary units.
The performance of MSOT-derived lipid signal in classifying PD patients and healthy volunteers was then assessed through single-classifier receiver operating characteristics (ROC) curve analysis and compared to the performance of body mass index and MRI-derived fat fraction values in the same task. Results indicate that MSOT-derived lipid signal performed better as a predictor of disease status, thus indicating that this metric may hold promise for diagnosing and monitoring muscle degeneration in patients with PD.
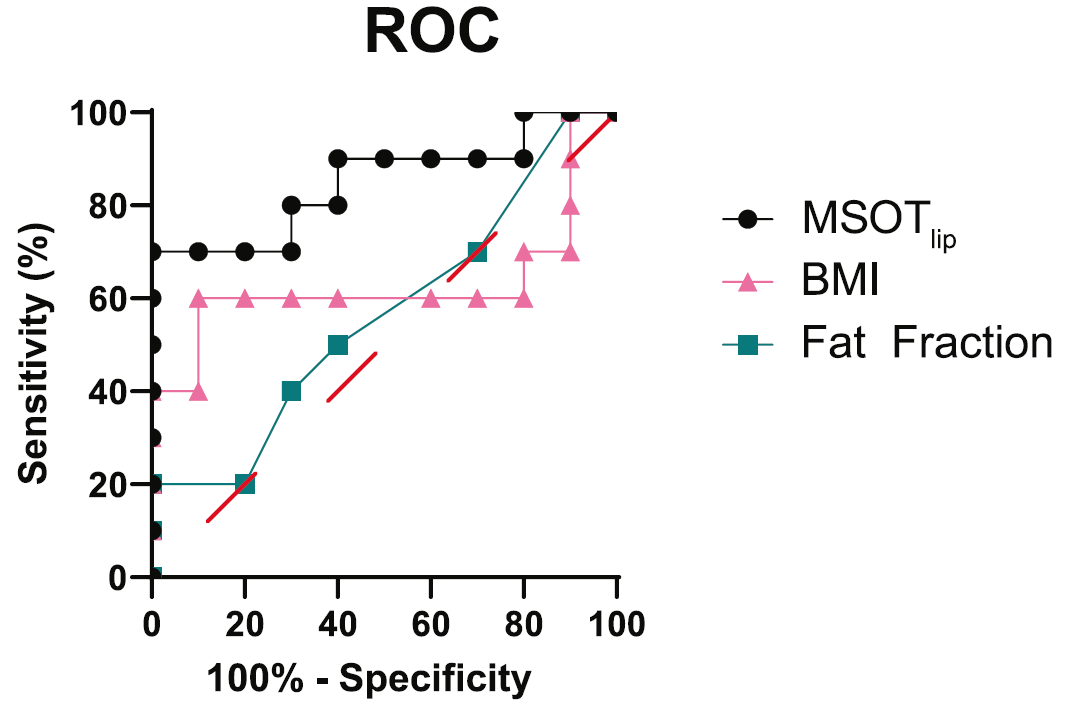
ROC curve comparing the performance of MSOT-derived lipid signal, body mass index (BMI) values, and the MRI-derived fat fraction values in distinguishing healthy volunteers from PD patients. Red dashes indicate the random classifier delineation.