False positives and false negatives: the need for better diagnostics
Early and accurate detection of breast cancer is invaluable to administer appropriate treatment, improve patient outcomes, and increase survival chances. In current clinical care, breast cancer screenings are typically performed with mammography, ultrasound, and MRI.
However, these approaches suffer from numerous drawbacks. The sensitivity of mammography strongly depends on the density of the imaged breast tissue, as cancerous lesions are often obscured by other dense features. Thus, mammography is prone to reporting false negatives, especially in younger women with typically denser breast tissue. An advanced form of mammography, digital breast tomosynthesis (DBT), has seen increased use in recent years. Although DBT has demonstrated increased sensitivity compared to standard mammography, it has not shown a significant increase in specificity. Additionally, mammography introduces ionizing radiation via low-energy X-rays into the imaged tissue, which itself could increase the risk of developing cancer in the future.
While ultrasound imaging offers a non-ionizing alternative to mammography and performs more reliably in dense breast tissue, this approach is also often hindered by lower specificity (i.e., reporting far more suspicious findings than later confirmed to be malignant) and high inter-operator variability. Finally, methods like MRI and molecular breast imaging have also been explored, although these come with significant costs and limited throughput.
Owing to the diagnostic gaps presented by existing imaging technologies, more precise (but invasive) methods like tissue biopsy and histological analyses are frequently necessary as follow-up tests to obtain a confident diagnosis. However, this may impart unnecessary burden on patients for whom initial screening results indicate a suspicious lesion that is subsequently down-graded as benign via histology.
Expanding the clinical imaging toolbox can improve diagnostic accuracy of breast cancer detection, thereby decreasing false negatives as well as false positives. Subsequent steps of disease staging and treatment response monitoring would also benefit from more sensitive yet non-invasive and non-ionizing tools by allowing for more frequent assessment with lower patient burden.
![]()
Disease patterns and functional markers imaged with MSOT
Cancerous cells require increased blood supply to fuel their rapid and unregulated growth. To that end, they induce the formation of new blood vessels in a process known as angiogenesis. Furthermore, these fast-growing tumors eventually outgrow this new blood supply as well, leading to hypoxia (i.e., reduced oxygenation) within the malignant tissue. Angiogenesis and hypoxia are some of the classic hallmarks of tumors, which means they can also serve as identifiable biomarkers of cancer.
Ongoing research at the cutting edge of biomedical imaging has led to the recent development of a new diagnostic imaging modality – multispectral optoacoustic tomography (MSOT). This non-invasive imaging technology visualizes the concentration and distribution of various clinically important biomolecules (such as oxygenated and deoxygenated hemoglobin), based on their natural light-absorbing properties.
In the context of assessing breast cancer, the unique advantage of MSOT is its ability to directly visualize the concentration and distribution of oxygenated and deoxygenated hemoglobin, including both high-resolution imaging of blood vessels as well as diffuse blood in soft tissue, thereby providing diagnostically relevant information about potential angiogenesis and hypoxia in and around a suspicious breast lesion. If integrated into clinical routine, MSOT has the potential to not only facilitate accurate diagnosis of breast lesions but also enable more frequent and quantitative assessment of treatment response and monitoring.
Several independent studies have been performed in recent years exploring the ability of MSOT to differentiate healthy breast tissue from cancerous tissue. In an initial pilot study, researchers at the Technical University Munich and the Helmholtz Center Munich utilized MSOT to assess breast cancer patients along with healthy volunteers. In particular, and with an eye towards detecting tumor blood supply as a biomarker of disease, they investigated the MSOT-derived signals from oxygenated and deoxygenated hemoglobin as well as total blood volume.
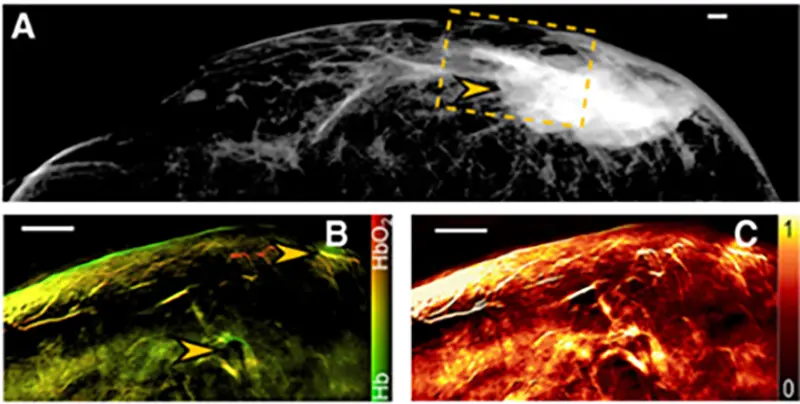
Multimodal imaging of a breast tumor. (A) Mammographic image of a breast tumor. The field of view imaged by MSOT (shown in B and C) is indicated by a dashed line box. (B) MSOT image of deoxygenated hemoglobin (Hb) and oxygenated hemoglobin (HbO2) obtained from the box indicated in A; arrows point to areas of increased blood volume. (C) MSOT image of total blood volume showing constitutive hyperemia through an extended area of the tumor. Clin Cancer Res. (2017)
MSOT imaging resolved a pattern of increased perfusion and blood vessels around the periphery of a tumor, while the core of the tumor showed reduced perfusion – a pattern that is strongly indicative of angiogenesis. Notably, these patterns were corroborated by MRI as well as histology but were not detectable with mammography or ultrasound imaging. Additionally, tumor areas exhibited highly variable total blood volume across the imaged area, which is a marked contrast from the uniform values observed in healthy volunteers. These results suggest that MSOT may be able to complement established breast cancer screening modalities by providing unique information on breast tissue vasculature as an indicator of disease.
More recently, another pilot study conducted at the University of Cambridge developed an optoacoustic imaging vascular feature set (i.e., a collection of archetypal vascular imaging patterns) to differentiate benign and malignant breast lesions. The participating patients had all presented with breast tissue abnormalities identified during prior screenings, which ranged from benign to suspicious and were formally diagnosed by histology. This pool of patients was then divided into two cohorts: a first cohort to derive the feature set, and a second cohort for validation.
The feature set was first derived by imaging of the breast vasculature using MSOT. These images were closely examined by experienced breast radiologists and researchers already experienced in optoacoustic imaging, who then aimed to correlate imaging patterns with the patient’s known diagnoses. The final feature set descriptions focused on patterns which were found to be unique to either benign or malignant lesions.
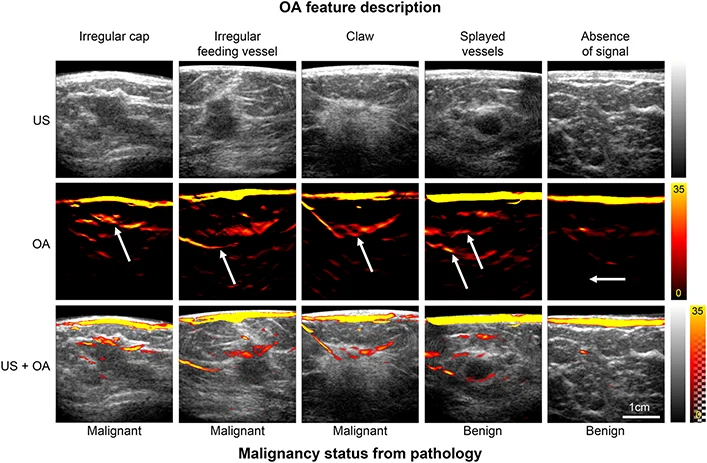
MSOT images illustrating the patterns used to derive the feature set for benign and malignant lesions. Optoacoustic (OA) features are specified above and lesion histopathology below the image panels. Arrows indicate the specific OA feature relative to the lesion, visible in the ultrasound (US) image and hybrid OA-US image. Photoacoustics (2022)
To validate the optoacoustic feature set, two additional radiologists were tasked with classifying lesions from the second cohort based on MSOT imaging data. Separately, they also classified the same patients according to only mammography and ultrasonography, which served to compare the performance of MSOT against the clinical gold standards. Notably, the validator radiologists were not involved in deriving the feature set and were blinded to the diagnosis of each patient.
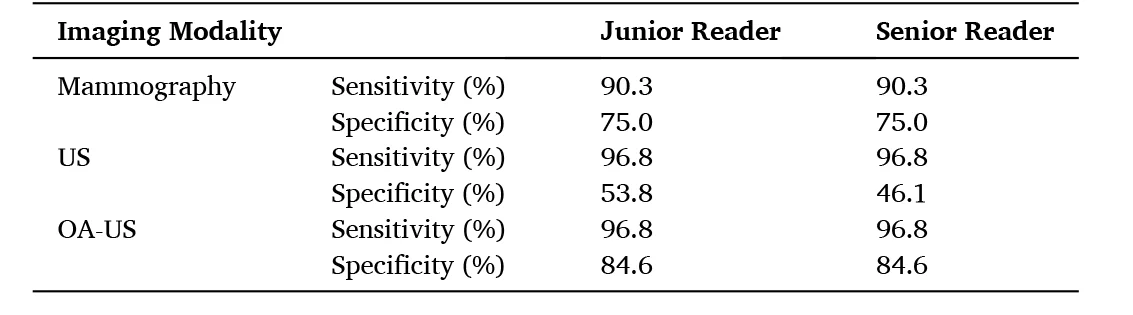
Summary of the sensitivity and specificity values for the standards-of-care mammography and ultrasound (US), compared to imaging with MSOT. Data adapted from: Photoacoustics (2022)
The performance of each method to correctly classify lesions as malignant was determined by the essential diagnostic metrics of sensitivity (the true positive rate) and specificity (the true negative rate). In terms of sensitivity, MSOT performed on par with ultrasound and better than mammography. Meanwhile, the specificity of MSOT was noticeably higher than both ultrasound and mammography, thereby reporting far fewer false positive findings and reducing the number of recommended breast biopsies.
The authors highlight that in all four instances where a false negative result was reported by ultrasound or mammography, assessment with MSOT was able to correctly report a true positive. Moreover, in three of these four false negatives reported by the clinical gold standards, breast density was determined to be high (a BI-RADS score of “C”). This is especially consistent with the tendency for the sensitivity of mammography to decrease with increasing breast density. However, since optoacoustic scoring was able to correctly classify all four of these lesions as malignant, the sensitivity of MSOT may be unaffected by tissue density.
Based on these results, the authors propose that imaging with MSOT and scoring according to the developed feature set could enhance the accuracy of breast cancer screenings in comparison to mammography and ultrasound alone. In particular, the demonstrated ability of MSOT to rule out cancer and thereby reduce unnecessary biopsies, related complications, and patient anxiety could bridge critical diagnostic gaps left by the clinical gold standards.
![]()