Medical science is constantly pursuing new, more effective means to treat and manage cancer. In particular, approaches that offer the ability to specifically target cancer cells while avoiding damage to healthy cells and simultaneously incurring lower systemic toxicity are especially attractive for clinicians and patients alike.
Within recent years, photothermal therapy (PTT) has arisen as a possible novel treatment method in diseases like cancer, where the ability to target specific cells is advantageous. In PTT, the subject or anatomical region of interest is injected with exogenous molecular agents (such as nanoparticles or conjugated small molecule dyes) that absorb light. When a light source is focused on the area targeted for treatment, the molecular agents enter an excited state and subsequently return to a ground state by releasing the absorbed light energy as vibrations and heat. This causes a sharp and highly localized spike in tissue temperature, which can trigger cell death in that specific area of the body.
As a potential novel method to combat cancer, PTT opens up the possibility to non-invasively cull tumor growth and potentially circumvent the toxic side-effects commonly induced by chemotherapeutic treatments.
![]()
The need for improved photothermal agents
For researchers aiming to advance PTT, a key challenge is a lack of photothermal molecular agents that are both efficacious and non-toxic. Numerous inorganic materials have been identified to have excellent photothermal conversion properties. However, these typically have poor biocompatibility and biodegradability, leading to concerns about their long-term safety for clinical use. On the other hand, while organic substances may present better biocompatibility profiles, they are typically far less efficient at converting photonic energy into the heat necessary for successful PTT.
Cyanine dyes, such as indocyanine green (ICG), are a group of fluorescent organic dyes widely employed in biomedical imaging due to their low toxicity and strong absorption in the near-infrared spectrum. However, these chromophores face several drawbacks for application in PTT, such as poor photostability and low photothermal conversion. Functionalization of cyanine dyes with new chemical structures offers the opportunity to improve their photothermal performance while taking advantage of their proven biocompatibility.
To that end, researchers at the Helmholtz Center Munich incorporated a barbiturate functional group into several cyanine structures and synthesized a set of three new barbiturate-cyanine dyes. The performance and properties of these dyes were assessed, and the best-performing molecule was further functionally enhanced to generate nanoparticles (BC1010-NPs). BC1010-NPs were then evaluated in an animal model for their potential as a new candidate agent for PTT.
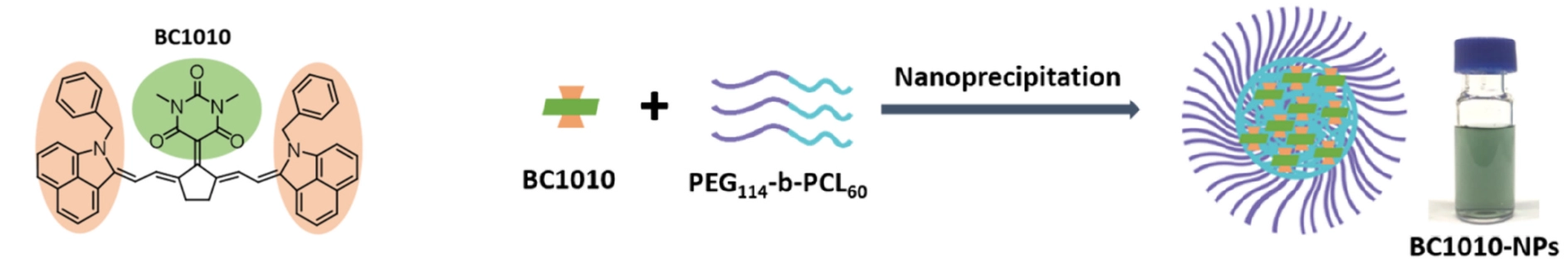
Left: Barbiturate-cyanine dye with peak absorption at 1010 nm (BC1010). Right: Further functionalization of BC1010 and nanoformulation of BC1010 nanoparticles (BC1010-NPs).Optoacoustic imaging reveals nanoparticle accumulation in tumors
One of the most crucial properties of PTT agents is that they localize in high concentrations in the targeted tissue of interest, rather than dispersing throughout the body or being cleared too rapidly for successful treatment. To assess the distribution of the developed nanoparticles in an in vivo setting, the tumor region of tumor-bearing mice was imaged pre-injection as well as at several timepoints post-injection with BC1010-NPs. Imaging was performed using Multispectral Optoacoustic Tomography (MSOT) to enable direct visualization of various light-absorbing chromophores – including the developed nanoparticles.
Following injection of BC1010-NPs, imaging with MSOT displayed a gradual signal increase of nanoparticles in the tumor region over time, with a maximum accumulation at approximately 12 h post-injection. Subsequently, optoacoustic imaging at 24 h showed a partial signal decrease, indicating the start of systemic nanoparticle clearance. Quantitative analysis of optoacoustic signals at the measured timepoints showed highest enrichment of BC1010-NPs within the tumor region at 4-12 h post-injection, suggesting that localization of BC1010-NPs in tumors is highest within this window and that this would be the optimal timeframe to perform PTT. These findings were corroborated by blood clearance profile measurements of BC1010-NPs, which indicate a nanoparticle clearance half-life of approximately 10 h.
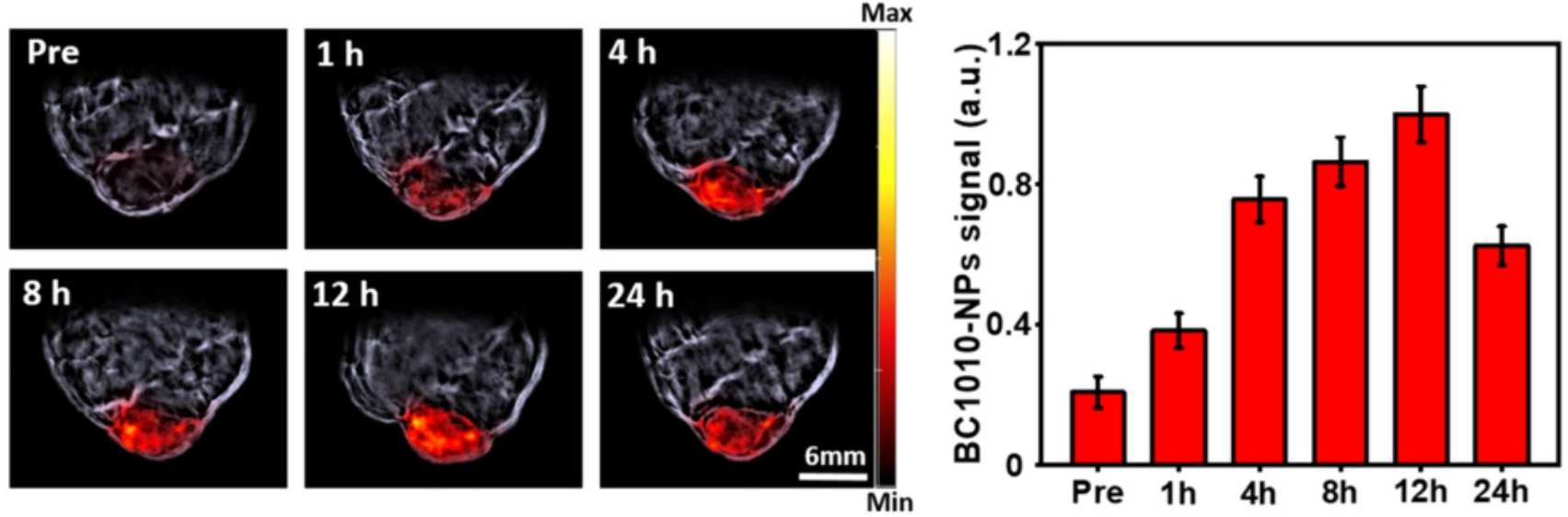
Left: Optoacoustic images of a subcutaneous tumor at various timepoints relative to injection of BC1010-NPs, showing signal from the nanoparticles and their local accumulation in the tumor region over time. Right: Quantitative analysis of MSOT signals in the tumor region over time.Efficient laser-triggered tumor elimination
To validate the potential of the nanoparticles as a novel agent for PTT, tumor-bearing mice were injected with either the BC1010-NPs or a negative control solution. Half of the mice from both treatment groups were then subjected to near-infrared laser irradiation of the tumor region, resulting in four treatment variations. Based on the time-resolved accumulation of BC1010-NPs measured earlier with MSOT, the laser-treated groups were irradiated 4 h post-injection, as this was determined to be the optimal timeframe to begin PTT.
In mice that had been both injected with the nanoparticles and received laser treatment, sharp temperature spikes up to ~50 °C were recorded in the tumor region, which is sufficient to induce the desired effect of targeted cell death. Most importantly, mice who received PTT showed significant tumor growth suppression in the days following treatment. Conversely, all control groups demonstrated rapid tumor growth over the same time period. These results were further confirmed via histological analysis of tumor tissue sections. Lastly, no significant toxic side-effects were detected in the mice who received PTT with BC1010-NPs.
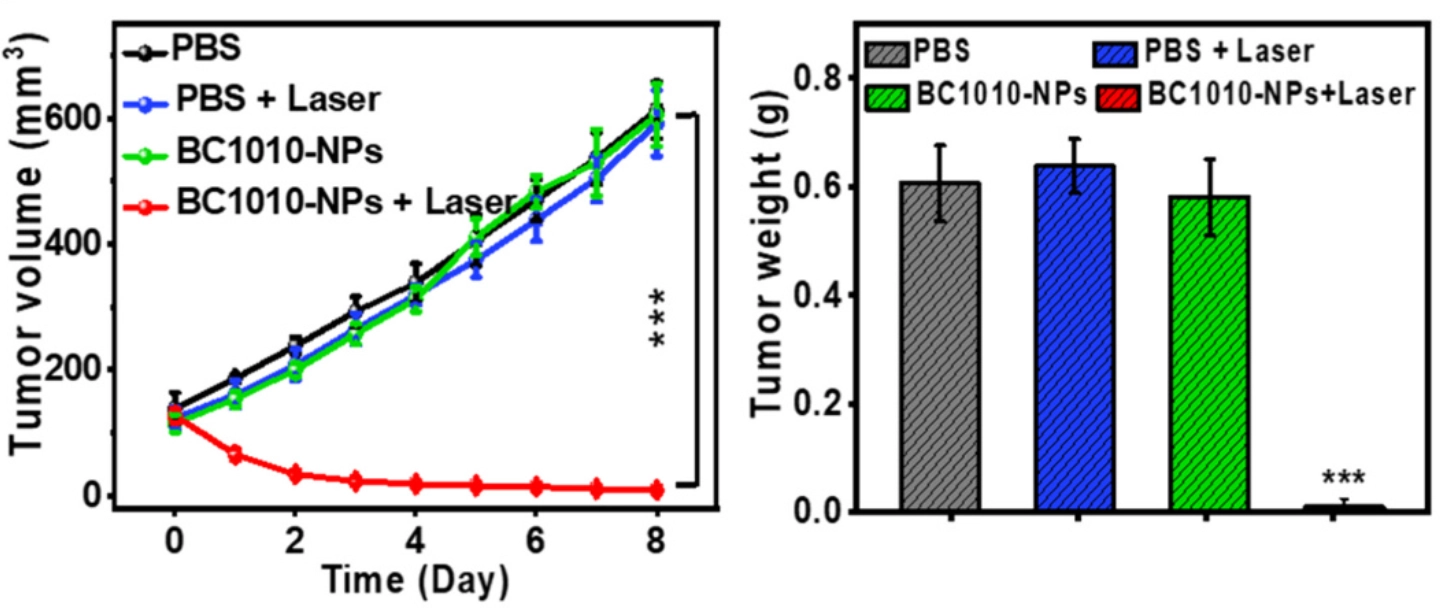
Tumor volume (left) and weight (right) of mice monitored over 8 days after injection of BC1010-NPs or PBS, with or without laser radiation.